▶ What is a Surfactant?
The word surfactant means surface active agent. Surfactants have a hydrophobic (water-hating) tail and a hydrophilic (water-loving) head.
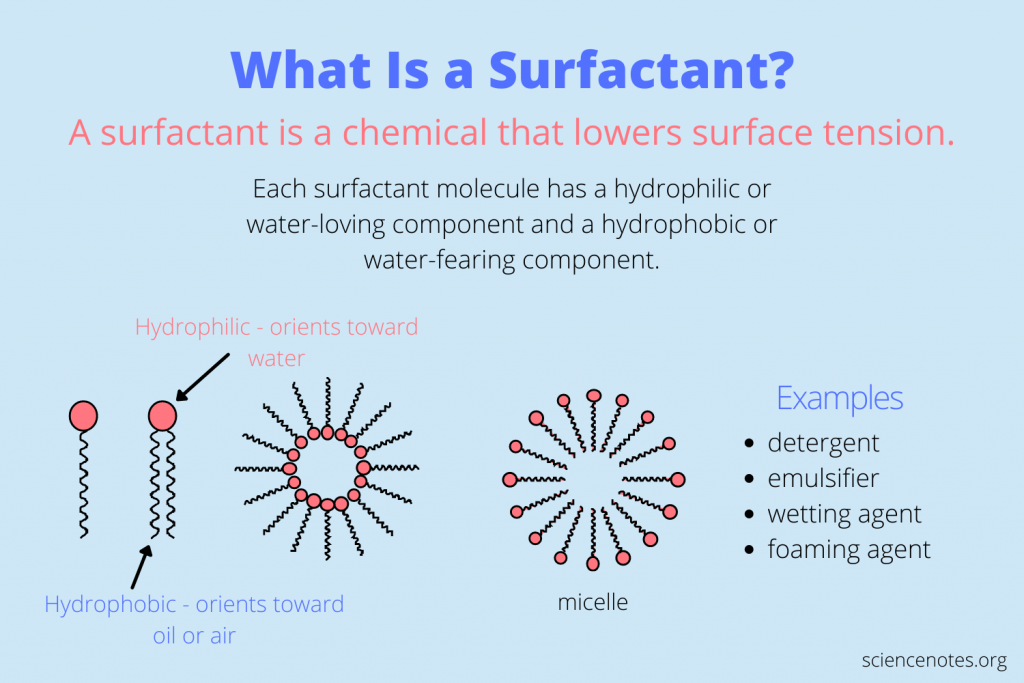
▶ How do surfactants work?
- When there are a sufficient amount of surfactant molecules present in a solution they combine together to form structures called micelles. As the micelle forms, the surfactant heads position themselves so they are exposed to water, while the tails are grouped together in the center of the structure protected from water.
▶ Types of Surfactants
- The hydrophilic head of each surfactant is electrically charged. The charge can be negative, positive, or neutral. Depending on the charge of the hydrophilic head, the surfactant is classified as anionic, nonionic, cationic or amphoteric.
▶ Anionic Surfactants
- Anionic surfactants have a negative charge on their hydrophilic end. Anionic surfactants create a lot of foam when mixed. Sulfates, sulfonates, and gluconates are examples of anionic surfactants.
▶ Nonionic Surfactants
- Nonionic surfactants are neutral, they do not have any charge on their hydrophilic end. Certain nonionic surfactants can be non-foaming or low-foaming.
- Nonionic surfactants have a unique property called a cloud point. The cloud point is the temperature at which the nonionic surfactant begins to separate from the cleaning solution, called phase separation. When this occurs, the cleaning solution becomes cloudy.
- The temperature of the cloud point depends upon the ratio of the hydrophobic and hydrophilic portions of the nonionic surfactant. Some cloud points are at room temperature while others are very high. Some nonionic surfactants don’t have a cloud point because they have a very high ratio of hydrophilic to hydrophobic moieties.
- Examples of some common nonionic surfactants include cocamide, ethoxylates, and alkoxylates.
▶ Cationic Surfactants
- Cationic surfactants have a positive charge on their hydrophilic end. The positive charge makes them useful in anti-static products, like fabric softeners. Cationic surfactants can also serve as antimicrobial agents, so they are often used in disinfectants.
- Cationic surfactants cannot be used with anionic surfactants. If positively charged cationic surfactants are mixed with negatively charged anionic surfactants, they will fall out of solution and no longer be effective. Cationic and nonionic surfactants, however, are compatible.
- Examples of some common cationic surfactants include alkyl ammonium chlorides.
▶ Amphoteric Surfactants
- Amphoteric surfactants have a dual charge on their hydrophilic end, both positive and negative. The dual charges cancel each other out creating a net charge of zero, referred to as zwitterionic. The pH of any given solution will determine how the amphoteric surfactants react. In acidic solutions, the amphoteric surfactants become positively charged and behave similarly to cationic surfactants. In alkaline solutions, they develop a negative charge, similar to anionic surfactants.
- Amphoteric surfactants are often used in personal care products such as shampoos and cosmetics. Examples of some frequently used amphoteric surfactants are betaines and amino oxides.
Source:




